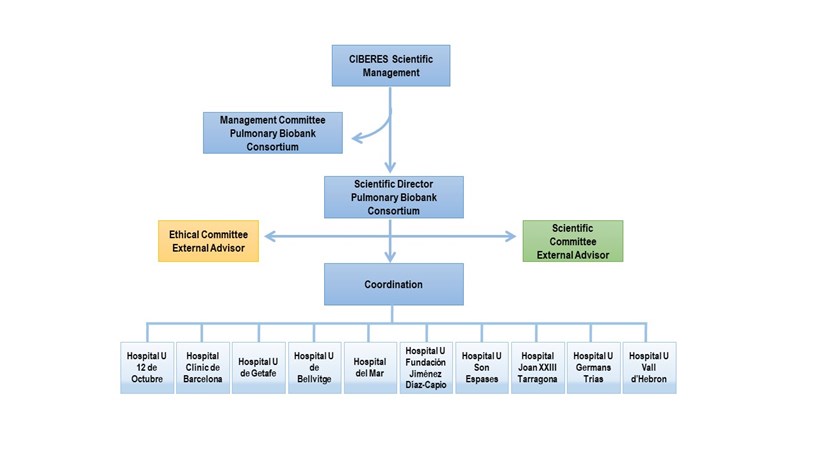
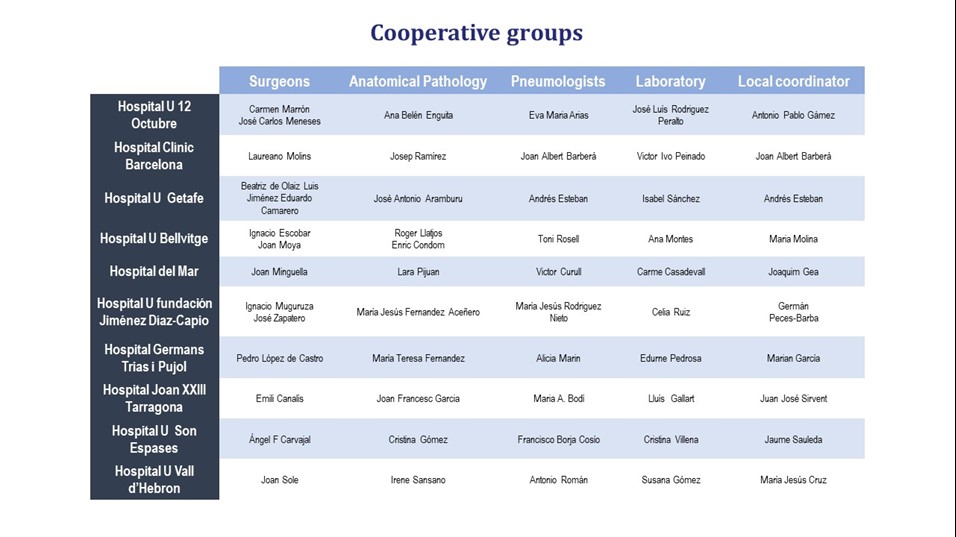
The Pulmonary Biobank Consortium (PBC) is a transversal, public and non-profit structure, promoted by the CIBER of Respiratory Diseases, where hospitals participate voluntary providing samples and relevant clinical information obtained from patients who come for care reasons (number B.0000471 National Registry of Biobanks - ISCIII). PBC was created in 2008 and its activity has been certified by the ISO9001:2015 standard (formerly ISO9001:2008) since 2012.
PBC coordinate different hospitals to:
- Cover the needs of researchers in terms of biological samples.
- Ensure the pre-analytical control of the samples to minimize artifacts and variations between batches of samples.
- Collaborate and participate in similar cooperative structures.
Its mission is to provide quality samples and data to national and international researchers, to promote reproducible translational biomedical research, and thus improve diagnosis and development of new treatments.
Also participates in:
- The ISCIII Biobanks and Biomodels Platform (https://www.isciiibiobanksbiomodels.es/)
- The PREDICTIVE MEDICINE Program of the Precision Medicine Infrastructure associated with Science and Technology (IMPaCT) https://www.isciii.es/QueHacemos/Financiacion/IMPaCT/Paginas/default.aspx
- Coordination and management of samples in national multicenter projects:
For more information click on the following link:
https://biobancopulmonar.ciberes.org/en